India can lead the global fight against counterfeit drugs
Every 10th medicine consumed in the world is either counterfeit or substandard (WHO). In India, that problem lies with every 6th medicine. With new-age Pharmaceutical Serialization and Track & Trace technology, the penetration of counterfeit drugs can reduce and patient safety across India and the world will improve.
Pharmaceutical Serialization (the process of assigning a unique identifier to each product unit) and Track & Trace are paramount to pharma companies' compliance with international regulations. Serial number duplication, tampering and rerouting of pharma drugs result in noncompliance. Noncompliance results not only in enormous fines but also a lower distributor score (e.g. US Distributor Scorecard’) leading to reputational damage. Legacy track and trace and compliance solutions use old architecture, are not fully automated, have limited flexibility and are expensive, making this sector ripe for disruption.
India's opportunity to lead by example
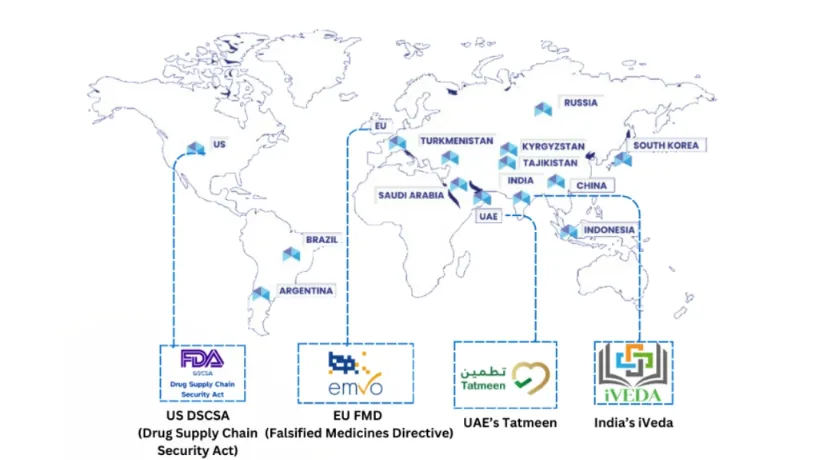
India has been exporting pharmaceutical products to more than 200 countries over 30 years. India’s pharma industry ranks third globally by volume and contributes ~1.7% to the nation's GDP. With export projections of $350 billion by 2047, pharma’s contribution to India’s current account deficit is well noted.
As a global leader in pharma production of generic drugs and vaccines, India possesses vast domain expertise and numerous pharma manufacturing plants. At the same time, it also possesses skilled tech talent and a successful history of home-grown SaaS companies selling to the globe.
This unique ecosystem positions companies and products born in India to build modern track and trace solutions that can adapt to the rapidly evolving market and spearhead the charge against the $200 billion global counterfeit drug market.
But what’s changing in the pharma serialization landscape? Where do the opportunities for improvement lie?
Old isn’t gold in pharma track & trace
Accuracy and speed are critical performance indicators in serialization and track & trace systems. Legacy systems, for all their market share dominance, fall short in both. The introduction of new compliance requirements, globally, demands rapid adaptation and better performance from track & trace systems.
We can observe the need for change in three fundamental areas: Cloud solutions, Automation capabilities and Analytics & AI.
Old architecture stifles compliance and is costlier
Legacy systems have moved, albeit at a snail’s pace, from on-premises solutions to cloud solutions, but at great cost to pharma companies.
The old architecture of legacy systems doesn’t allow easy migration of data. Merging multiple databases is impractical and faces severe scaling challenges, particularly when dealing with decades of data. This increases a company’s compliance expenses. For example, a pharma company based in the USA and India might have to expend on two separate track & trace software licences, as opposed to one unified system, due to challenges in matching and verification of tens of terabytes of data using the old architecture.
New-age systems with advanced database architecture and front-end architecture can hasten the shift to cloud solutions. This will lead to more reports generated per minute and better compliance.
Newer systems can also host (privately) each pharma company on the company’s own cloud infrastructure, contrary to the multi-tenant systems deployed by legacy players. This difference could have a positive exponential impact on post-production data upload speed, and consequently, on swift compliance with multi-national regulations.
Automation capabilities must match the dynamic nature of compliance
With every new regulation, the format of reporting changes. Legacy tools operate using proprietary data exchange formats, converting a universally accepted format into their format and reconverting it back to the original. This process results in significant errors, which are more prominent when new regulations kick in. Moreover, legacy systems cannot move to new formats as decades of data are stored in an old format. Legacy systems take weeks to resolve errors as they utilise a ticket-based service system, operated by their backend support to identify errors.
Modern systems can resolve such errors in a few hours, without external reliance and at a lower cost. A pharma company can internally establish an operator-led error correction system that flags errors by itself and automate independently.
Typically, a compliance report’s file size is 100 MB. However, legacy tools can handle only 20 MB at a time, compelling a person to manually break down a report into five parts. This exercise only compounds the number of human hours consumed.
New-age tools can process large file sizes and operate on the latest data formats with the ability to adapt and update formats every time a regulation is introduced. Modern systems must enable a pharma company to design its own workflows and be self-reliant.
Copilots and AI-driven analytics will improve real-time compliance, and lower the cost of compliance
Building a Gen-AI tool as a knowledge repository that answers compliance queries could add immense value to pharma compliance automation. Not only will the regulations buried deep inside regulatory agency websites become easily available, but also the charges “per compliance query” that legacy players charge can be side-stepped.
A great starting point to introduce copilots can be to solve for real-time regulations updates. With auto-updates and human-in-the-loop mechanisms, copilots for data exchange format updates can quickly incorporate required changes into the serialization and track & trace systems with greater accuracy.
Analytics on production data, reject rates, production speed, and so on, are currently offered at large, one-time fees in the form of a generic dashboard that pharma companies may or may not use. With AI-driven analytics, granular data can be analysed, and customers can pay on a usage-based billing structure.
Clear gaps and opportunities exist in the track & trace landscape, across markets and industries
In large markets, such as Indonesia and Africa, pharma track & trace and automated compliance remain unsolved by incumbents. Expected changes in policy and global regulations will likely open more new markets for Track & Trace providers in emerging economies.
The Food & Beverage industry faces similar challenges that the pharma industry does. Likely adopters would be higher margin industries, the likes of alcohol and tobacco, where counterfeits are high in number and track & trace regulations are still in an elementary form.
Forward Actions against Counterfeit Drugs
Modern pharma serialization, track & trace systems must be built to smoothen workflows and compliance processes in pharma. Successful and swift compliance at each node of the value chain is imperative to guarantee patient safety.
The question beckons – how does it lead to global change? We can chart two paths that lead to widespread adoption.
Firstly, the challenges with existing systems present an opportunity for a leading track & trace and compliance product from India. The complex and rapidly changing regulatory environment acts as a key catalyst.
Pharma companies in India can become early adopters and spearhead the wave of modernizing pharma track & trace. Companies with an Indian as well as an overseas presence, reporting to various regulated countries, can drive the deployment of new track & trace tools across multiple continents.
Secondly, with the help of regulatory bodies and governments, adoption by domestic downstream supply chain participants can follow suit at a national scale - safeguarding transparency and supply chain resilience all the way to small-time distributors and pharmacies.
The promise of new age serialization and track & trace solutions is as serious the $200 Bn problem presented by counterfeit drugs. Pharma companies can avoid fines, cut revenue losses with more efficient compliance tools and most importantly, guarantee patient safety.